Lymphatics:
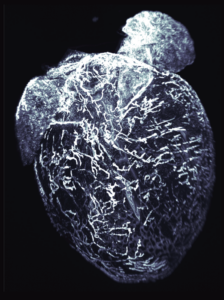
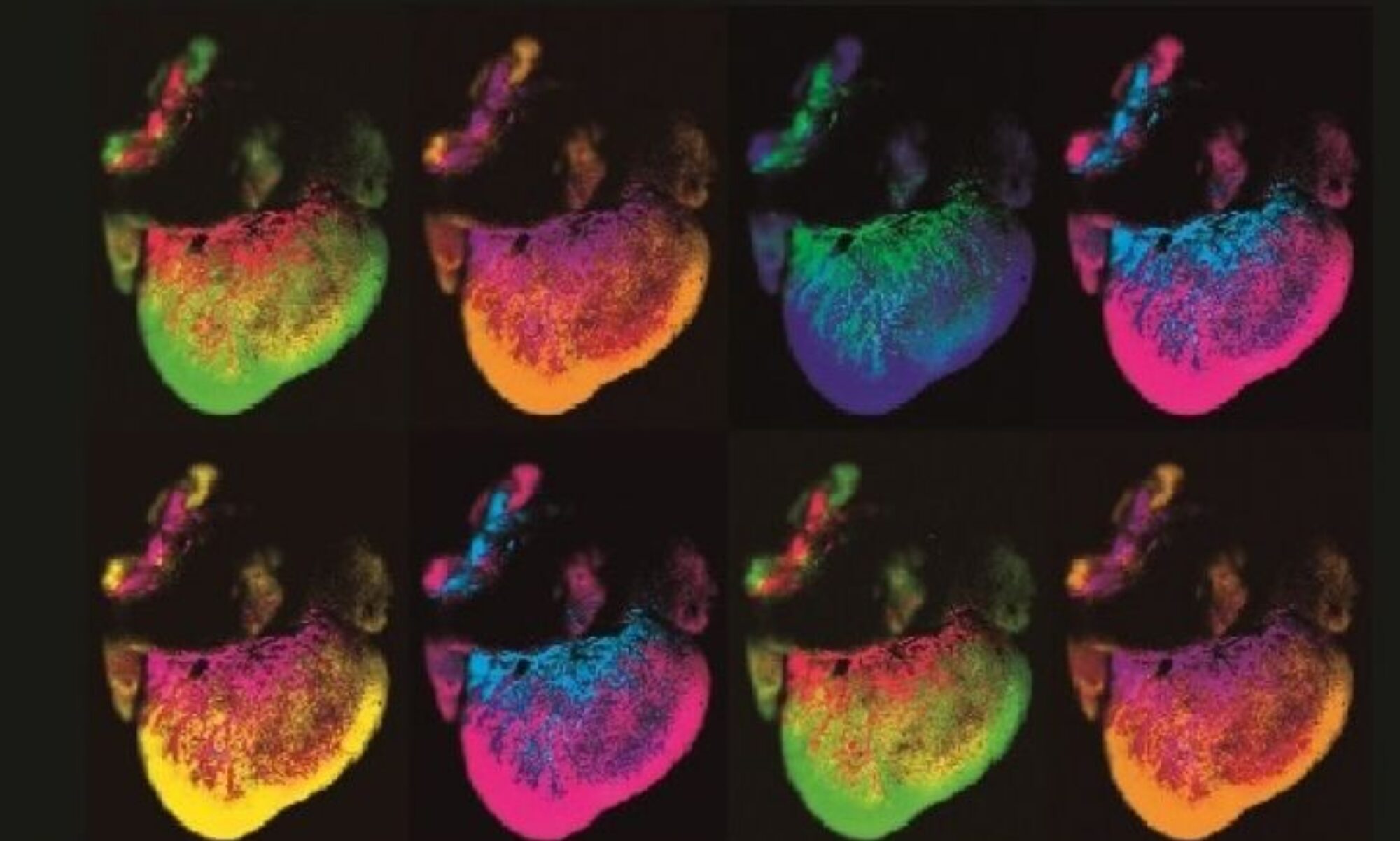
We have pioneered the study of G protein-coupled receptor (GPCR) pathways, including orphan, chemokine and Family B receptors, in the development and function of the lymphatic vascular system. Lymphatics play essential roles in maintaining appropriate interstitial fluid balance, trafficking immune cells, and absorbing dietary fat within the gastrointestinal tract. Our most recent work using spatiotemporal gene engineering approaches permits us to compare and contrast the differences in lymphatic development and function in a variety of peripheral organs, like the gastrointestinal tract and heart—an area of broad and comparative investigation that has been largely understudied in the field to date. We strive to identify pharmacologically-tractable lymphatic GPCRs, elucidate the genetic and signaling pathways that govern sex differences in lymphatic physiology, and characterize the molecular basis of lymphatic vessel flow and permeability.
Receptor Activity Modifying Proteins:
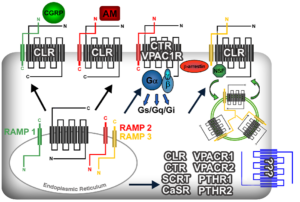 RAMPs are a small class of GPCR accessory proteins that chaperone receptors to the cell surface and impart allosteric regulation of ligand binding specificity, functional selectivity and intracellular trafficking for a variety of GPCRs. RAMPs, first identified in the context of the adrenomedullin receptor, represent a unique paradigm for GPCR functional regulation which is currently being exploited by the pharmaceutical industry. Our laboratory was the first to generate and characterize genetic mouse models null for each of the 3 Ramp genes. Phenotypic characterization of these models has permitted us to functionally validate the requirement and roles of RAMPs for controlling the signaling of many family B GPCR ligands, including adrenomedullin, CGRP, VIP and CRF. Most importantly, we have leveraged these genetic tools with in vitro biochemistry and pharmacology approaches to expand the current repertoire of RAMP-interacting GPCRs, thereby providing novel pharmacological targets for the modulation of a wide variety of pathophysiological conditions including skeletal wasting, hypothalamic-pituitary imbalance, gut-brain axis regulation, asthma and cardiac failure. Our current studies are geared towards characterizing the pharmacological and physiological roles of RAMPs with several newly identified partners in the chemokine/cytokine GPCR subfamily.
RAMPs are a small class of GPCR accessory proteins that chaperone receptors to the cell surface and impart allosteric regulation of ligand binding specificity, functional selectivity and intracellular trafficking for a variety of GPCRs. RAMPs, first identified in the context of the adrenomedullin receptor, represent a unique paradigm for GPCR functional regulation which is currently being exploited by the pharmaceutical industry. Our laboratory was the first to generate and characterize genetic mouse models null for each of the 3 Ramp genes. Phenotypic characterization of these models has permitted us to functionally validate the requirement and roles of RAMPs for controlling the signaling of many family B GPCR ligands, including adrenomedullin, CGRP, VIP and CRF. Most importantly, we have leveraged these genetic tools with in vitro biochemistry and pharmacology approaches to expand the current repertoire of RAMP-interacting GPCRs, thereby providing novel pharmacological targets for the modulation of a wide variety of pathophysiological conditions including skeletal wasting, hypothalamic-pituitary imbalance, gut-brain axis regulation, asthma and cardiac failure. Our current studies are geared towards characterizing the pharmacological and physiological roles of RAMPs with several newly identified partners in the chemokine/cytokine GPCR subfamily.
Reproductive Biology:
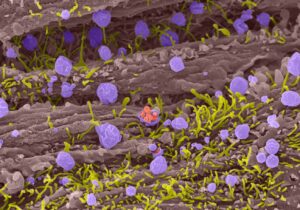
The precise genetic dosage of adrenomedullin, both from the mother and the fetus, is crucial for establishing and maintaining a normal pregnancy. Female mice with a modest 50% reduction in adrenomedullin gene expression suffer from subfertility due to a variety of reproductive defects including abnormal uterine receptivity, implantation and placentation. Moreover, loss of adrenomedullin from fetal tissues leads to pathological features of preeclampsia and placental edema in the placentas of mice and humans. Through our extensive and productive collaborations with clinician scientists and the pharmaceutical industry, we have translated our discoveries to humans, with the hopes of developing therapeutic approaches for assisted reproductive technologies. Our studies are contributing to the basic understanding of molecules and processes that govern maternal-to-fetal communication in the placenta and have the potential of providing new clinical diagnostic tools and therapeutic approaches for the amelioration of complications of infertility and pregnancy.